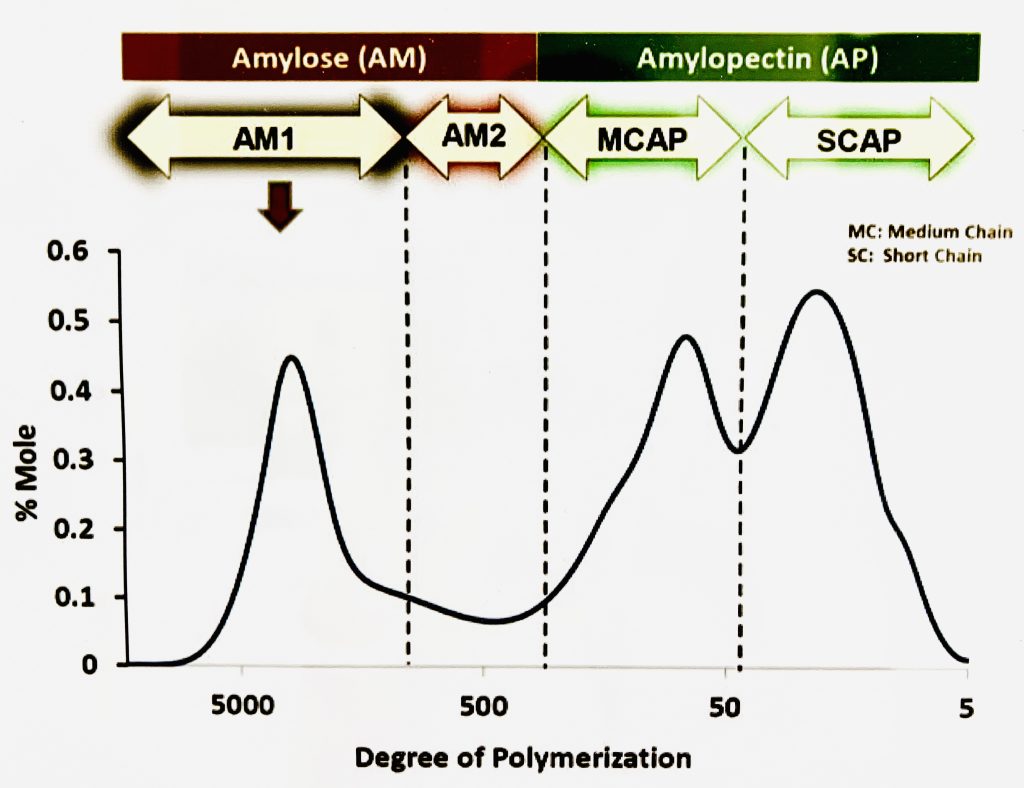
This week, Jazz and I analyzed data from previous SEC experiments. SEC, or size exclusion chromatography, is a technique that separates molecules based on their size. A sample passes through a column containing a gel with pores. Smaller molecules get trapped in these pores while larger molecules continue to move down through the column. Thus, larger molecules are eluted out of the machine first. The refractive index (RI) detector then quantifies the length of each chain. A graph can be made that separates each molecule based on its degree of polymerization (DP), which is the amount of monomer units in a polymer.

Starch is made up of 2 glucose polymers: amylose and amylopectin. Amylose has a mostly linear structure while amylopectin is very branched. The amount of each polymer affects the cooking and eating quality of rice. Rice with higher amounts of amylose tends to be harder while more amylopectin results in gelatinous and sticky rice. When conducting SEC, the starch is debranched enzymatically so all the amylopectin branches are separated. This results in amylopectin having a lower DP than amylose. Amylose elutes before amylopectin because its larger coils are able to move faster through the column while the smaller amylopectin coils are trapped in the pores.
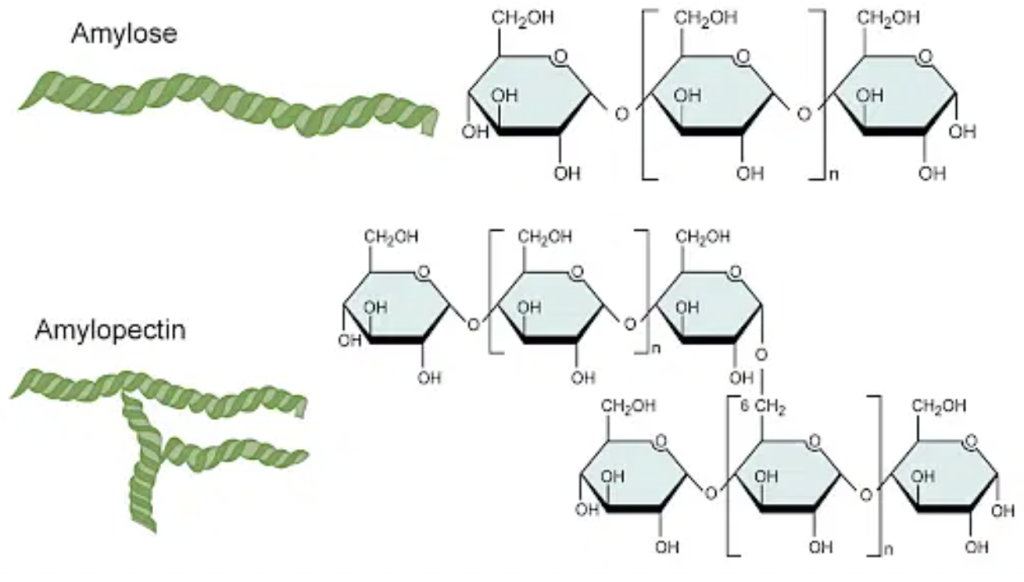
To find the total amount of amylose and amylopectin in a sample, the area under the curve is calculated when graphing % mole against the degree of polymerization. Jazz and I were given raw data with retention times and milimoles of starch, which we had to convert to the percentage of amylose and amylopectin in the samples. I will explain more details about the actual experiment in a future blog post!
Later this week, Jazz and I also conducted another GI experiment, the same one we did my first week. There were 15 pre-weighed samples of rice in large test tubes. We then added water to each tube and cooked the rice in a boiling pot of water. Next, we prepared buffer solutions with their respective enzymes (amylase, pepsin, AMG/pancreatin). There is a certain time table we follow to add the enzymes and acid/base into the tubes, which takes a total of 1 hour and 32 minutes to finish. This is to replicate the time your food is introduced to each enzyme. The first enzyme added is amylase, which is found in the saliva. It breaks down starch into maltose (2 glucose units). Then, the rice is minced using a spatula to replicate chewing. After that, HCl is added to copy the acidic environment of the stomach. Pepsin is then added to break down proteins into smaller peptides. Next, the pH is increased by adding NaOH to copy the environment of the pancreas and intestines. An aliquot is taken to represent the amount of glucose before complete digestion (t=0). Then, a combination of amyloglucosidase (AMG) and pancreatin are added. Pancreatin contains trypsin, amylase, and lipase, which break down protein, starch, and lipids, respectively. AMG imitates the activity of maltase, glucoamylase, sucrase, and isomaltase in the small intestine. Thirty minutes after these enzymes are added, another aliquot is taken (t=30). After centrifuging, making more aliquots, adding AMG, mixing, incubating, adding GOPOD, and incubating once again, we transferred the samples to a microplate and placed them into a UV/vis spectrometer to receive absorbance readings. Using correction factors, dilution factors, the starch constant, and total volume, we can convert the absorbances to milligrams of starch. This is then converted to percent of starch hydrolyzed. Next, we find the area under the curve between the percent of starch hydrolyzed at t=0 and t=30 and use a correction factor to find what the estimated GI value is. In this run, the GI values were in the fifties, which is considered low to intermediate.

Outside of the lab, we had an eventful week! On Tuesday, the girls had a relaxing self-care night where we did face masks and jammed out to music. Wednesday was another holiday (Eid al-Adha) so we had another day off! At first, the IWU group went to Robinsons to do some clothes shopping. Then, we went to Siento Cafe, which had really good passionfruit slushies! Afterwards, we played volleyball at IRRI and became completely soaked in sweat. On Thursday, the scholars program at IRRI hosted a “social hour” where someone gives a presentation about the country of the month. This month, a couple presented the geography and culture of Pakistan. We played volleyball again on Friday, but this time, a lot of locals joined us so it felt like a real game. Throughout this week, Julia slept over in my dorm because there was an infestation of cockroaches in her room (there were only 3).

On Saturday, the IWU interns and a few other IRRI interns hiked the peak of Mount Makiling. As mentioned in a previous post, Mount Makiling is a dormant volcano that has an elevation of 3,580 ft. I have never been hiking before so this was quite the first time experience! We woke up early in the morning, grabbed breakfast at McDonalds, and headed over to the mountain by using a jeepney. There are 2 hikes you can do: one on a gravel road to the base of the summit and one from the base to the peak. We skipped over the gravel road and started from the base by taking motorcycles up the road. Our group had a decently quick pace, and we reached the summit in 2.5 hours. On the way up, there we parts where we had to climb ladders and use ropes to get up because of the near 90º inclines. It felt like I was in some outdoor survival show! There were also a lot of tiny blood-sucking leeches that stuck onto our pants. Luckily, they were fairly easy to remove as we just pinched them or flicked them off. After taking pictures and eating snacks at the peak, we headed back to the base. I think I had a harder time coming back down because it was so slippery! It took us a little over 2 hours on the hike back. Thankfully, we all survived with no huge wipeouts or injuries! Later that evening, Hunter (Manila) and Sam (Santo Tomas) came over so we showed them a few of our favorite spots in Los Baños.



